

The CMV transmission parameters and congenital disease risks are well established, 9, 10, 11, 12 even though details of transmission parameters and the worldwide distribution of this disease have only recently come to light.
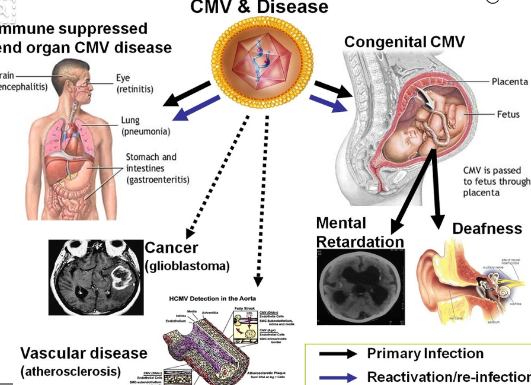
Several recent perspectives 1, 2, 3, 4, 5, 6, 7 and modeling efforts 8 provide thoughtful background on cytomegalovirus (CMV) transmission via mucosal contact with infected body fluids as well as the desirability of a CMV vaccine. This review focuses on the current understanding of natural and CMV vaccine-induced protective immunity. Together with CMV vaccine candidates currently in clinical development, additional promising preclinical strategies continue to come forward however, these face limitations due to the insufficient understanding of host defense mechanisms that prevent transmission, as well as the age-old challenges of reaching the appropriate threshold of immunogenicity, efficacy, durability and potency. Efficacy studies must be expanded to mixed populations of CMV-naive and naturally infected subjects to understand the overall efficacy and potential. Despite these hurdles, early phase clinical trials have achieved primary end points in CMV seronegative subjects. Immunity resulting from natural infection appears to limit rather than prevent reactivation of latent viruses and susceptibility to re-infection, leaving a challenge for universal vaccination to improve upon natural immunity levels. Although current vaccine strategies recognize the value of humoral and cellular immunity, the precise mechanisms that act at the placental interface remain elusive.

Given that a majority of females worldwide experience CMV infection during childhood, a universal vaccine must boost natural immunity and reduce transmission due to reactivation and re-infection as well as primary infection during pregnancy.

Children and adolescents are the targets for universal vaccination, with the expectation of protecting the offspring of immunized women. Reducing the complexity of such trials would facilitate vaccine development. Vaccine researchers, industry leaders, and regulatory representatives have discussed the challenges posed by clinical efficacy trials that would lead to a universal CMV vaccine, reviewing the links between infection and disease, and identifying settings where disrupting viral transmission might provide a surrogate endpoint for disease prevention. Thus, a vaccination against this virus has long been recognized for the potential of enormous health-care savings because congenital damage is life-long and existing anti-viral options are limited. An effective vaccination that prevents transplacental transmission would reduce CMV congenital disease and CMV-associated still births and leave populations less susceptible to opportunistic CMV disease. A universal cytomegalovirus (CMV) vaccination promises to reduce the burden of the developmental damage that afflicts up to 0.5% of live births worldwide.


 0 kommentar(er)
0 kommentar(er)
